Abstract
Background: Older patients (pts) with relapsed/refractory multiple myeloma (RRMM) represent a particularly difficult-to-treat population due to comorbidities, frailty or reduced fitness level, and treatment with concomitant medications (Larocca et al. Leukemia. 2018;32:1697); further, pts may have limited therapeutic options due to ineligibility to receive more intensive treatments (eg, autologous stem cell transplant [ASCT]). Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate that targets aminopeptidases and thereby rapidly releases alkylating agents into tumor cells. Melflufen + dexamethasone (dex) showed clinically meaningful efficacy and a manageable safety profile in pts with heavily pretreated RRMM in the phase 2 HORIZON study (OP-106; NCT02963493; Richardson et al. J Clin Oncol. 2021;39:757), which led to its accelerated approval in the United States. In the phase 3 OCEAN study (OP-103; NCT03151811; Oncopeptides. Press release. July 8, 2021), a head-to-head comparison of melflufen + dex and pomalidomide (pom) + dex showed superiority of melflufen (vs pom) with a 39% longer median progression-free survival (PFS) (HR, 0.79 [95% CI, 0.64-0.98], P=0.0311), but no clear overall survival (OS) benefit. This analysis compares the efficacy between pts in the melflufen arm vs pom arm based on age and prior ASCT in the OCEAN study.
Methods: Pts with RRMM had received 2-4 prior lines of therapy (LoTs), including lenalidomide (len) and a proteasome inhibitor, and were refractory to both the last LoT and len (within 18 mo of randomization). Stratified by age (≥75 y vs <75 y), number of prior LoTs, and International Staging System (ISS) score, pts were randomized 1:1 to 28-d cycles of melflufen 40 mg IV on d1 or pom 4 mg orally (PO) daily on d1 to 21. All pts received dex 40 mg (20 mg for pts ≥75 y) PO on d1, 8, 15, and 22. Pts received therapy until disease progression or unacceptable toxicity. The primary endpoint was PFS of melflufen vs pom, assessed by an independent review committee per International Myeloma Working Group Uniform Response Criteria. Key secondary endpoints included overall response rate (ORR) and OS. A post-hoc analysis was conducted in different age groups, based on prior ASCT.
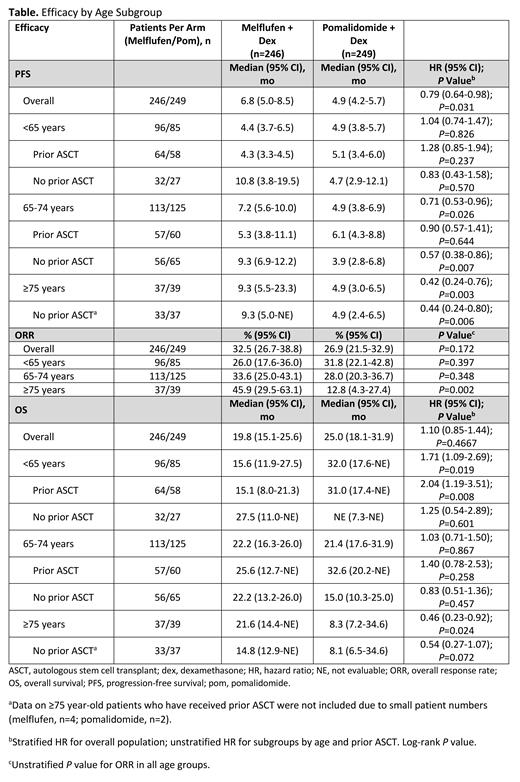
Results: As of February 3, 2021, 495 pts were randomized (n=246, melflufen; n=249, pom); 96 (39%) in the melflufen arm and 85 (34%) in the pom arm were aged <65 y, 113 (46%) in the melflufen arm and 125 (50%) in the pom arm were aged 65-74 y, and 37 (15%) in the melflufen arm and 39 (16%) in the pom arm were aged ≥75 y. Baseline characteristics, including prior ASCT, were well balanced between the 2 arms for each age group. Median PFS was 4.4 mo vs 4.9 mo in age <65 y, 7.2 mo vs 4.9 mo in age 65-74 y, and 9.3 mo vs 4.9 mo in age ≥75 y, with melflufen vs pom, respectively, with significant differences in the 65-74 y and ≥75 y groups (Table). In pts who had not received prior ASCT, median PFS was 10.8 mo vs 4.7 mo in age <65 y (HR, 0.83 [95% CI, 0.43-1.58]; P=0.57), 9.3 mo vs 3.9 mo in age 65-74 y (HR, 0.57 [95% CI, 0.38-0.86]; P<0.01), and 9.3 mo vs 4.9 mo in age ≥75 y (HR, 0.44 [95% CI, 0.24-0.80]; P<0.01), with melfluflen vs pom, respectively. Median OS was 15.6 mo vs 32.0 mo in age <65 y, 22.2 mo vs 21.4 mo in age 65-74 y, and 21.6 mo vs 8.3 mo in age ≥75 y, showing a trend for better OS with melflufen + dex with increasing age and a significantly longer OS in the ≥75 y group (HR, 0.46 [95% CI, 0.23-0.92]; P<0.05). ORRs were 26%, 34%, and 46% in the melflufen arm and 32%, 28%, and 13% in the pom arm, for <65 y, 65-74 y, and ≥75 y groups, respectively, indicating a trend for greater ORR with increasing age in the melflufen arm. The safety of melflufen + dex was consistent in all age groups. Serious treatment-emergent adverse events (TEAEs) were reported in 33% vs 44% in age <65 y, 45% vs 49% in age 65-75 y, and 54% vs 41% in age ≥75 y in with melflufen vs pom, respectively. TEAEs led to dose modifications (delay/reduction) of melflufen/pom in 79%/55% in age <65 y, 82%/59% in age 65-74 y, and 65%/64% in age ≥75 y, and discontinuation of melflufen/pom in 26%/20% in age <65 y, 28%/20% in age 65-74 y, and 22%/33% in age ≥75 y.
Conclusion: In the OCEAN study, melflufen + dex showed efficacy in pts aged ≥65 y with a more favorable PFS and numerically higher ORR and OS; this effect was pronounced in pts who had not previously received ASCT. Taken together, these data support further evaluation of melflufen + dex in older pts with RRMM, particularly in those who have not previously received ASCT.
Mateos: Oncopeptides: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; GSK: Honoraria; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Robak: Janssen: Honoraria; Celgene: Honoraria, Research Funding; Amgen: Honoraria; Medical University of Lodz: Current Employment. Rosiñol: Janssen, Celgene, Amgen and Takeda: Honoraria. Symeonidis: BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Demo: Research Funding; WinMedica: Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GenesisPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Research Funding; Sanofi/Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sonneveld: Janssen: Consultancy, Honoraria, Research Funding; SkylineDx: Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Thuresson: Oncopeptides AB: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Byrne: BMS: Current holder of individual stocks in a privately-held company; Oncopeptides AB: Current Employment, Current holder of stock options in a privately-held company. Harmenberg: Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses .
This abstract includes a subgroup analysis of a phase 3 investigational study of melflufen in patients with RRMM refractory to lenalidomide.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal